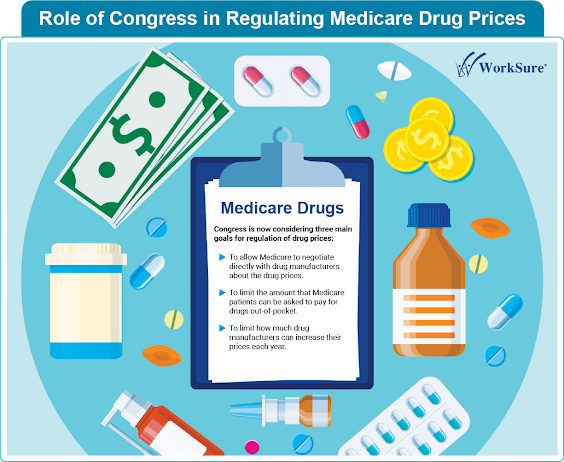
Role of Congress in Regulating Medicare Drug Prices Drug developmen t is an uncertain and expensive process, but there are various ways to lower the risk of failure and the expense of innovation without allowing drug corporations to charge outrageous prices. For example, in 1981, Congress established tax credits to offset the costs of research, and in 2000, Medicare began covering medical expenses for people participating in clinical trials. More recently, measures have been enacted to expedite drug approval by the Food and Drug Administration (FDA), potentially saving hundreds of millions of dollars for the firms. #drugdevelopment #clinicaltrials #clinicaldatamanagement #clinicaldocumentation #medicalwriting #medicalcoding Read more at : https://lnkd.in/dG-5-_Pn Our Services: https://lnkd.in/gip88kZc