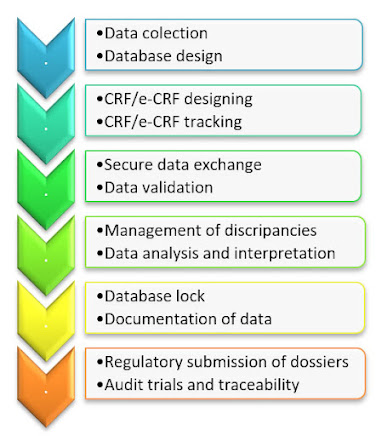
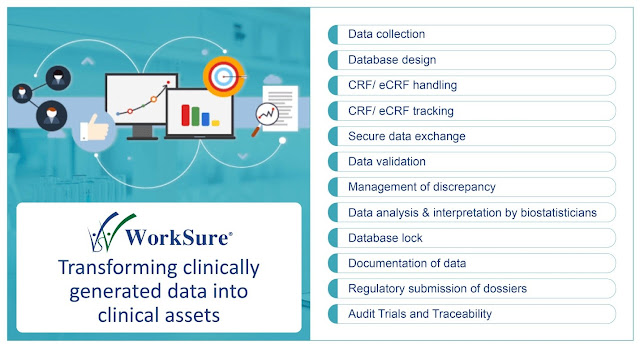
HEALTH DATA INSIGHTS THAT ENABLE CLINICAL OUTCOMES

Clinical data isn’t the only area to check for useful information to improve patient care outcomes. There is a need to use consumer demographic and lifestyle data in ways that assist the healthcare sector shift its focus from delivering sick care to working with people (rather than ‘patients’) to help them stay healthy in order to substantially improve health care. Read more at https://lnkd.in/dbxzYu6u Check our service at https://lnkd.in/gCNXRAz #clinicaldatamanagement #clinicalresearch #clinicaltrials #clinicalstudies #clinicaldevelopment #clinicaloperations