WAY OF TRANSFORMING CLINICAL DATA INTO MEANINGFUL INFORMATION BY CLINICAL DATA MANAGEMENT
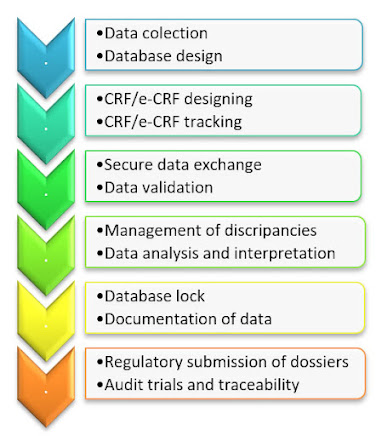
Clinical data management (CDM) is the process of gathering and maintaining study data in line with regulatory criteria in order to acquire high-quality, error free information. CDM is the process of establishing a protocol for a clinical research. CDM is thought to be a critical phase in clinical research since it leads to the creation of high, dependable, and statistically sound data, which helps to shorten the period between drug development and marketing. It entails entering, verifying, validating, and quality-controlling data collected during the course of a clinical research. Regulatory bodies all over the world rely on pharmaceutical companies participating in clinical trials adhering to quality standards and providing reliable and accurate clinical data.
When designing the protocol for the clinical trial, the Clinical Data Management (CDM) is kept in mind from the start.
Read more at: http://www.worksure.org/clinical-data-management-transforming-clinical-data-meaningful-information/

Comments
Post a Comment